ALVION PHARMACEUTICALS
A science oriented pharmaceutical company
Developing and manufacturing noninfringing generic medicines in Europe.
About us
Alvion is a science oriented pharmaceutical company developing and manufacturing non infringing generic medicines in Europe. Collaborating with state of the art R&D centers in EU, Alvion is a private and independent company focusing in R&D, IP, Quality, Regulatory and manufacturing of generic and added value medicines, delivering an exceptional track record in the global pharmaceutical market supplying millions of patients.
Why Alvion?
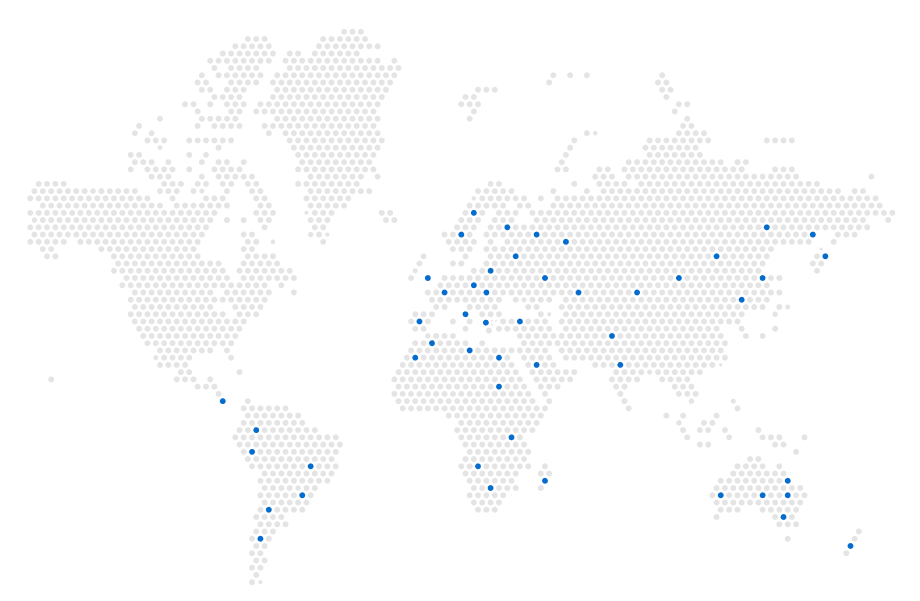
Developing, manufacturing, and promoting medicines in Europe, MENA, Canada, Australia, South Africa, Australia, Asia and Latin America is the core business model of Alvion. Focusing on delivering innovation and best quality pharmaceuticals among the first companies prior the patent expiry while offering flexible terms and affordable medicines with an effective supply chain is major competitive advantage of Alvion.
Our Vision
Alvion is a science pharmaceutical company offering difficult to develop and non-infringing products to its partners, setting a new standard in generics by combining our experiences with an understanding of the health care needs in a global level.
Our Mission
Our Goal
is to become a leading and vertically integrated pharmaceutical company, establishing a global presence, serving the healthcare communities and satisfying high performance employees.
our partners’ success is our success
By raising the barrier of customer service and innovation, Alvion’s team is developing and producing blockbuster molecules, registering the products across a wide territory. Alvion is offering a complete and competitive “package” to its partners delivering end to end products and services at all stages of the partnership.

Partner with Alvion
Innovation with a Global Presence
At Alvion, we are rethinking pharmaceuticals with a new business model that creates the pharmaceutical company of tomorrow. We strive for a change that is clearly visible in the various pharmaceutical markets, our model, processes and actions.

Research and Development
Developing in third parties’ high technology and non-infringing Intellectual Properties, often patented technologies, Alvion is an IP powerhouse providing licensing rights to the largest pharmaceutical companies in the world.
Products
Our people are a core aspect of Alvion’ s competitive advantage. We attract and retain key talents with a high level of expertise and know-how who drive our business forward.
Innovations equals successful business
Alvion is welcoming business opportunities with multinational and strong local pharmaceutical companies partnering with Alvion’s products via licensing agreements, both in the proprietary and the supply.

Career
We are looking for talents. Should you wish to work with one of the most dynamic teams in the industry, please share your CV with us at career@alvionpharma.com
Code of conduct
Alvion is committed to fair and vigorous competition because this is essential to ensure appropriate and fair market and even more so to ensure full access to affordable healthcare. Alvion does not tolerate any breach or possible infringement of any competition laws and regulations in any of its businesses.
Geographic global presence
Alvion is committed to fair and vigorous competition because this is essential to ensure appropriate and fair market and even more so to ensure full access to affordable healthcare. Alvion does not tolerate any breach or possible infringement of any competition laws and regulations in any of its businesses.